If you are pursuing an IND License, we have biologics manufacturing time slots available to manufacture your investigational product for use in clinical trials.
coming in the future: Interbiome Drug Shortage Division

cGMP manufacture of commercial drugs "abandoned" by pharma firms, yet still required for rare (orphan) & off-label patient needs, including oncology drugs of last resort (Small-molecule drugs as well as biologics)
If there is an "abandoned" drug you wish to retain access to, please contact us. We want to know about your present and future On-Label and Off-Label needs, and we will help arrange commercial manufacturing of the small-molecule or biologics product you need.
If there is an "abandoned" drug you wish to retain access to, we can arrange commercial manufacturing of the small-molecule or biologics product you need
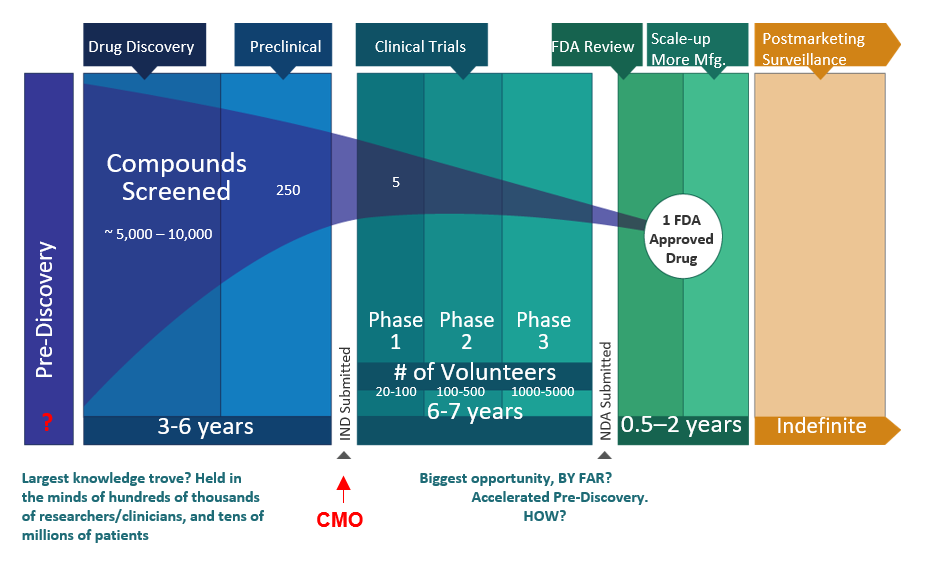
Lagging throughput. Rife with Fallacies of Scale. Therapies cost too much (>$2Bil/drug), and take too long (~15 yr). The net process is plagued with uncertainties, so quality is expensive to manage.
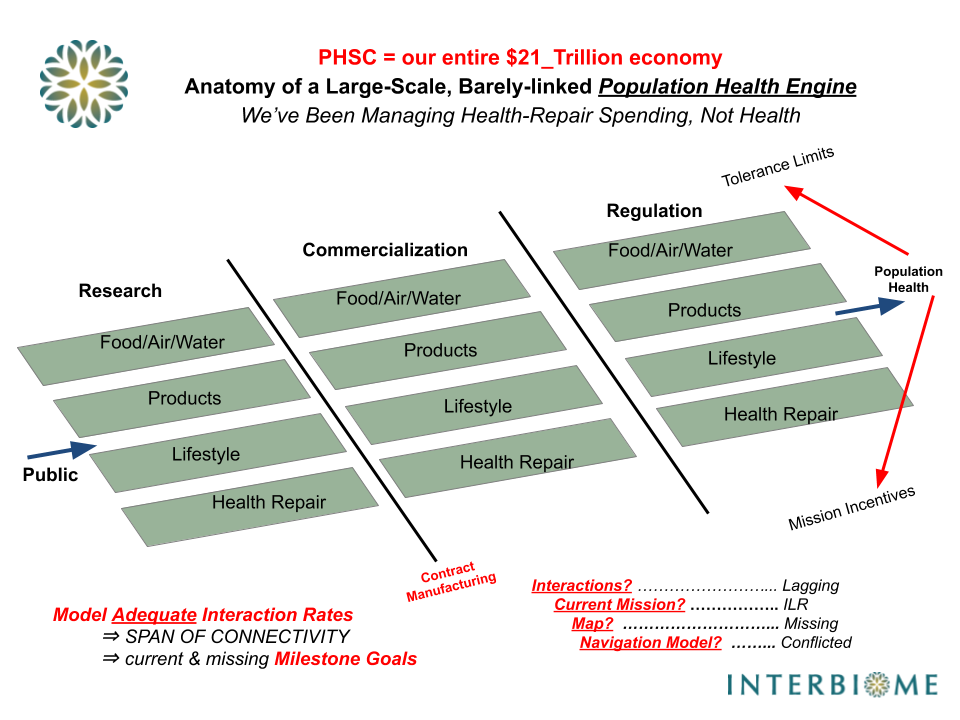
Only solution? Teamwork. Far richer feedback network, more interactions and more coordination. i.e., Biology-101 AND Industry-101